HELIOPHORE Project
© Meletios Verras
Hemiindigoids as visible-light-induced optical switches for photopharmacological regulation
Keywords
Photopharmacology, hemiindigoids, Alzheimer’s disease, acetylcholinesterase, visible light, optical control, photoswitch

Summary
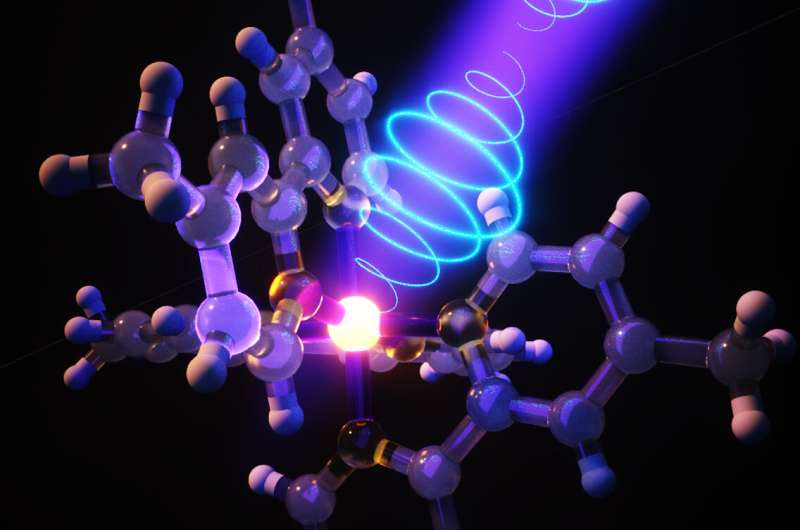
Photopharmacology offers a compelling approach to precisely control the bioactivity of drugs in terms of both location and timing. It relies on photoswitches, which are molecular structures capable of interconverting between two isomeric forms when exposed to light of particular wavelengths. Ideally, this system involves only one biologically active isomer that can be reversibly formed on demand in specific body area and timeframe. Thus, photopharmacology represents a complementary approach to other phototherapeutic strategies, like photodynamic therapies or photodecagingbased prodrugs. However, since its inception fifteen years ago, photopharmacology has almost exclusively utilized azobenzenes as photoswitches. Indeed, this molecular scaffold presents some properties that are particularly interesting in this context, such as a high change in terms of molecular geometry and polarity upon isomerization. However, the UV light required for their E→Z isomerization is often low-penetrating and potentially harmful, making them less suitable for in vivo and clinical applications. Hemiindigoids, i.e., hemiindigos (HI) and hemithioindigos (HTI), present a promising alternative. They can interconvert using visible light in both directions, with Z→E isomerization typically occurring in the 450–470 nm range and E→Z in the 530–650 nm range. Recent fundamental research has shed light on the global properties of HI and HTI.
Project HELIOPHORE aims to apply these photoswitch compounds to photopharmacology, by (1) deciphering the photoswitching properties of Hemiindigoid systems in water and physiological media through systematic series of molecules studied experimentally and theoretically, a blind spot of the existing studies, (2) designing Hemiindigoid inhibitors of acetylcholinesterase — an enzyme involved in Alzheimer’s disease (AD) —, with isomer-dependent activity, by relying on a very encouraging preliminary prototype, and (3) translate the in vitro potential to in vivo outcomes through a progressive pipeline of assays involving complex biological media, human cells, zebrafish larvae and transgenic AD mice, with a constant focus on the dark-vs.-light activity.
Consortium
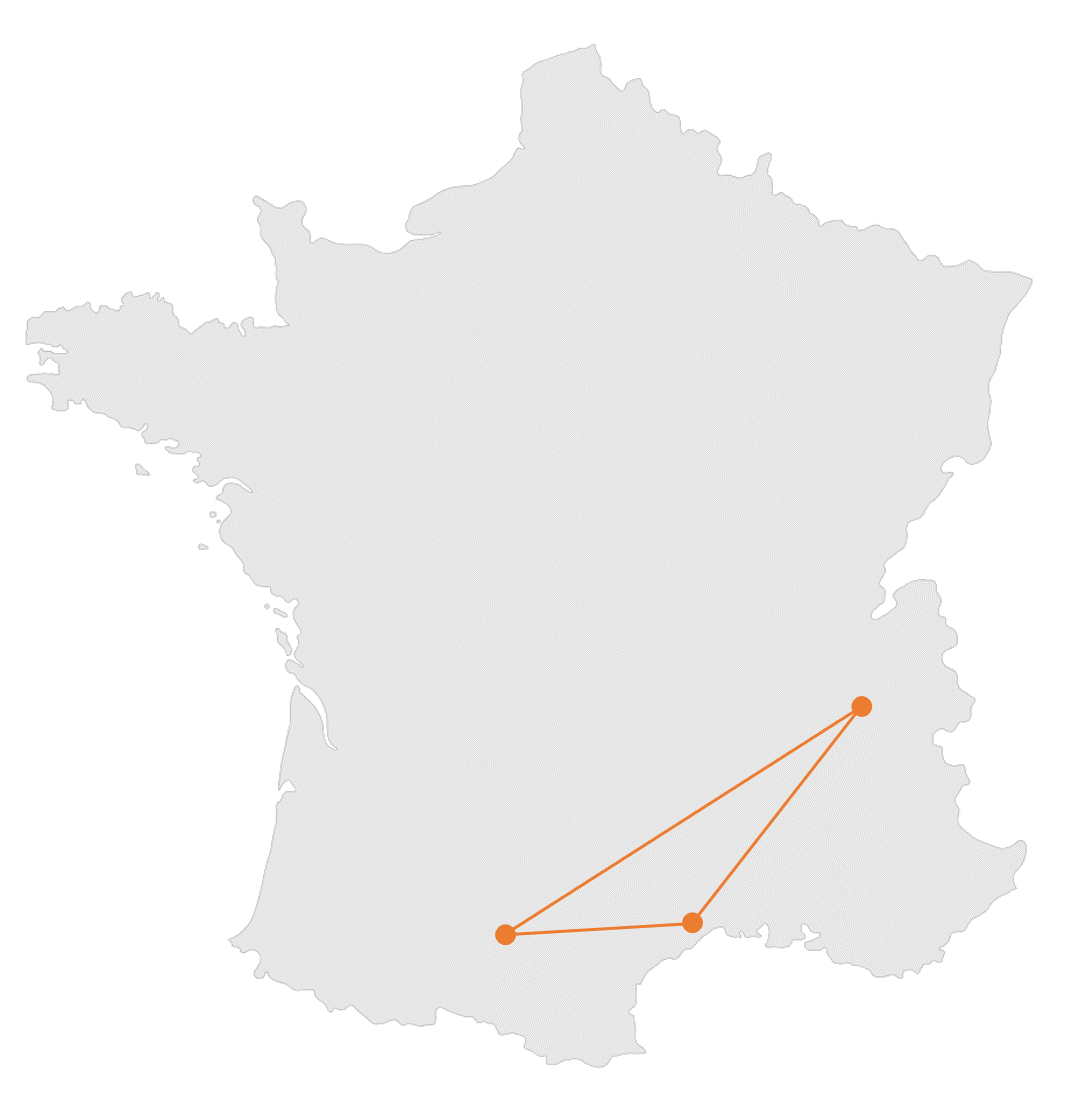
DPM • Grenoble
Département de Pharmacochimie Moléculaire
Romain Haudecoeur, Mickael Henry
DCM • Grenoble
Département de Chimie Moléculaire
Frédérique Loiseau, Damien Jouvenot
LCPQ • Toulouse
Laboratoire de Chimie et de Physique Quantiques
Martial Boggio-Pasqua, Marie-Catherine Heitz
IGF • Montpellier
Institut de Génomique Fonctionnelle
Cyril Goudet, Laurent Givalois, Fanny Ferreux, Guillaume Lebon, Damien Maurel, Chris Jopling, Aurélien Drouard, Magalie Mathias






Publications
Journal articles
- Letícia da Mata Lazinski, Morane Beaumet, Frédérique Loiseau, Cyril Goudet, Martial Boggio‐pasqua, et al.. Bistable and Water‐Operable Hemiindigo Photoswitches Allow Optical Control of Acetylcholinesterase Activity with Visible Light. ChemistryEurope, 2025, 3 (5), pp.e202500221. ⟨10.1002/ceur.202500221⟩. ⟨hal-05339073⟩
Les autres projets PEPR