HEALTH
© Meletios Verras
Light and molecular targets for phototherapies of tomorrow
Keywords
Clinical applications; nanoparticles; ultrafast optics; photodiagnostics; photomedicines; photosensitizers; photodynamic therapies; photothermal therapies; cancer treatment
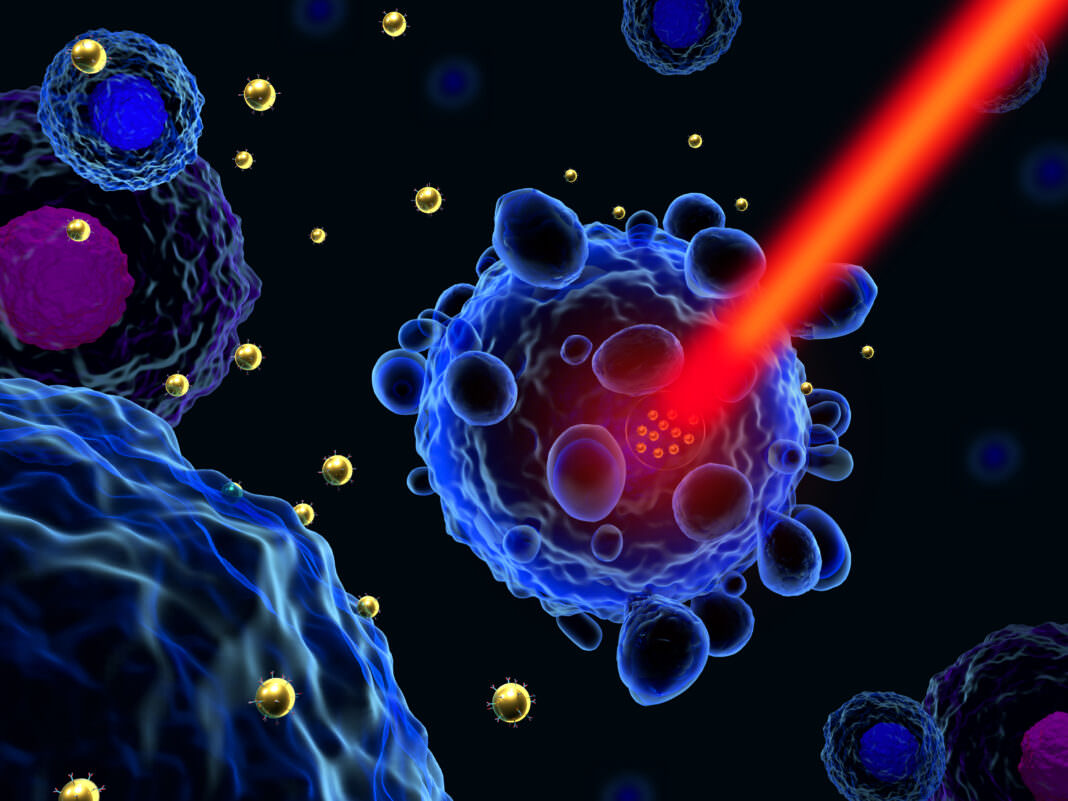
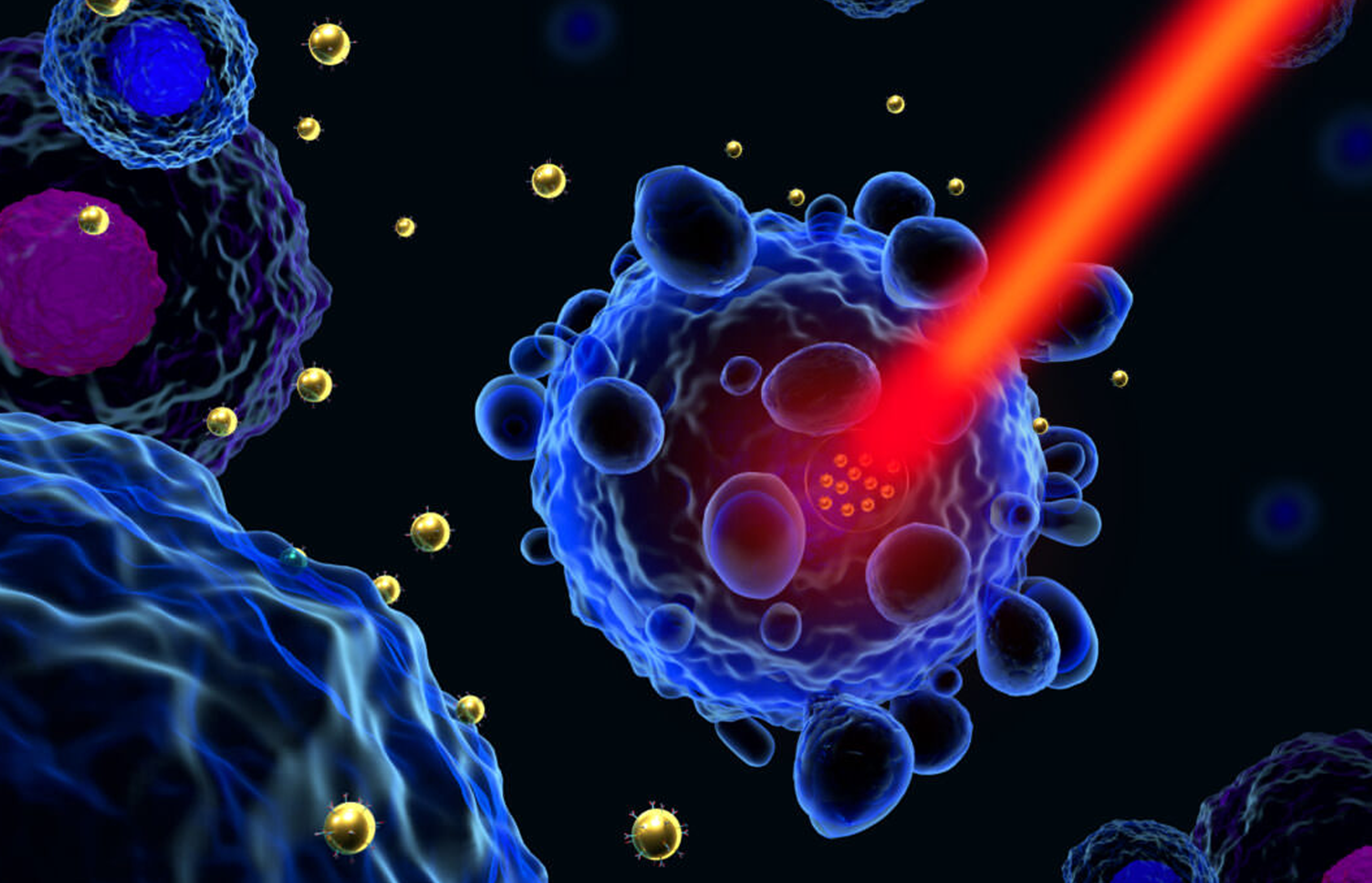
Light can help detect and heal. However, despite several therapeutic successes, phototherapies are currently used only for niche applications. Creating the conditions for high efficacy, low side effect phototherapies requires innovative tools and protocols with the highest degree of spatial and temporal control.
The key idea is to effectively combine the design of photomedicines and the control of ultrafast light beams to achieve real therapeutic breakthroughs. Using light-activatable molecular-scale structures, such as nanoparticles (NPs) or molecules, seems promising to achieve this goal. It will first require the synthesis of functionalized objects with specific or combined light-induced properties. It will also demand access, both in the laboratory and at the clinical level, to suitable light/radiation sources capable of triggering photoinduced processes at will by these objects. Finally, understanding the physical, chemical, and biological responses at multiple scales will allow us to optimize treatments.
Scientific objectives
Designing non-conventional photosensitizing objects
We will benefit from the active French nanomedicine community to design biocompatible photodrugs with targeting capabilities and differential responses concerning diseased tissues. This knowledge will be combined with input from materials science and photophysics to consider multiple ways for these objects to interact with light, such as high two-photon and X-ray absorption cross sections, photon upconversion (for efficiency in deep tissues), photodynamic and photothermal actions (for efficiency in oxic and anoxic tissues), light-induced drug release.
Defining new strategies to optimize phototherapies using advanced characteristics of light
Light sources could provide new ways to control the photoactivation process using sophisticated combinations of light pulse sequences, polarizations, coherent control, spatiotemporal beam patterns, etc. It will make it possible to control the light-matter interaction, define where the energy is deposited, and know how fast and in what degrees of freedom the energy is transferred and dissipated, optimizing phototherapeutic effects, achieving high penetration depths, and circumventing therapeutic evasion.
Understanding microscopic steps leading to a therapeutic action following light absorption to minimize side effects and optimize efficiency
Time-resolved recordings from the picosecond to the
attosecond timescales provide detailed information on the basic mechanisms of the photoactivation process by measuring charge dynamics, secondary emissions, energy flows, and structural rearrangements at the microscopic scale. A better understanding of the post-light absorption processes will allow us to optimize the actuation process. It will also allow controlling the influence of the way light energy is deposited (pulse duration, repetition rate) on the biological effects (radical formation, temperature rise, pressure). Then, the key question is how the biological system will respond to this stress (apoptosis, necrosis, vasoconstriction, induction of acute local inflammatory response, and activation of the immune system).
In this goal, we aim to reach the preclinical stage for several cancer types with no ideal treatment and low life expectancy after diagnosis (glioblastoma, mesothelioma, pancreatic cancer, etc.). We will tailor the targeting here (e.g., NRP-1 for glioblastoma or ELP for tumor inflammatory environment) to address these diverse situations.
Development of prototypes based on compact advanced light sources for targeted treatments in clinical applications
This requires proof-of-principle demonstrations using LUMA’s Ultrafast infrastructure, synthesis, modeling, and links with French companies in the field of ultrafast optics. It also requires a scale-up of synthesis and normalization of illumination procedures between sites to prepare for the clinical step.
The projects involved

Pas d’actualités